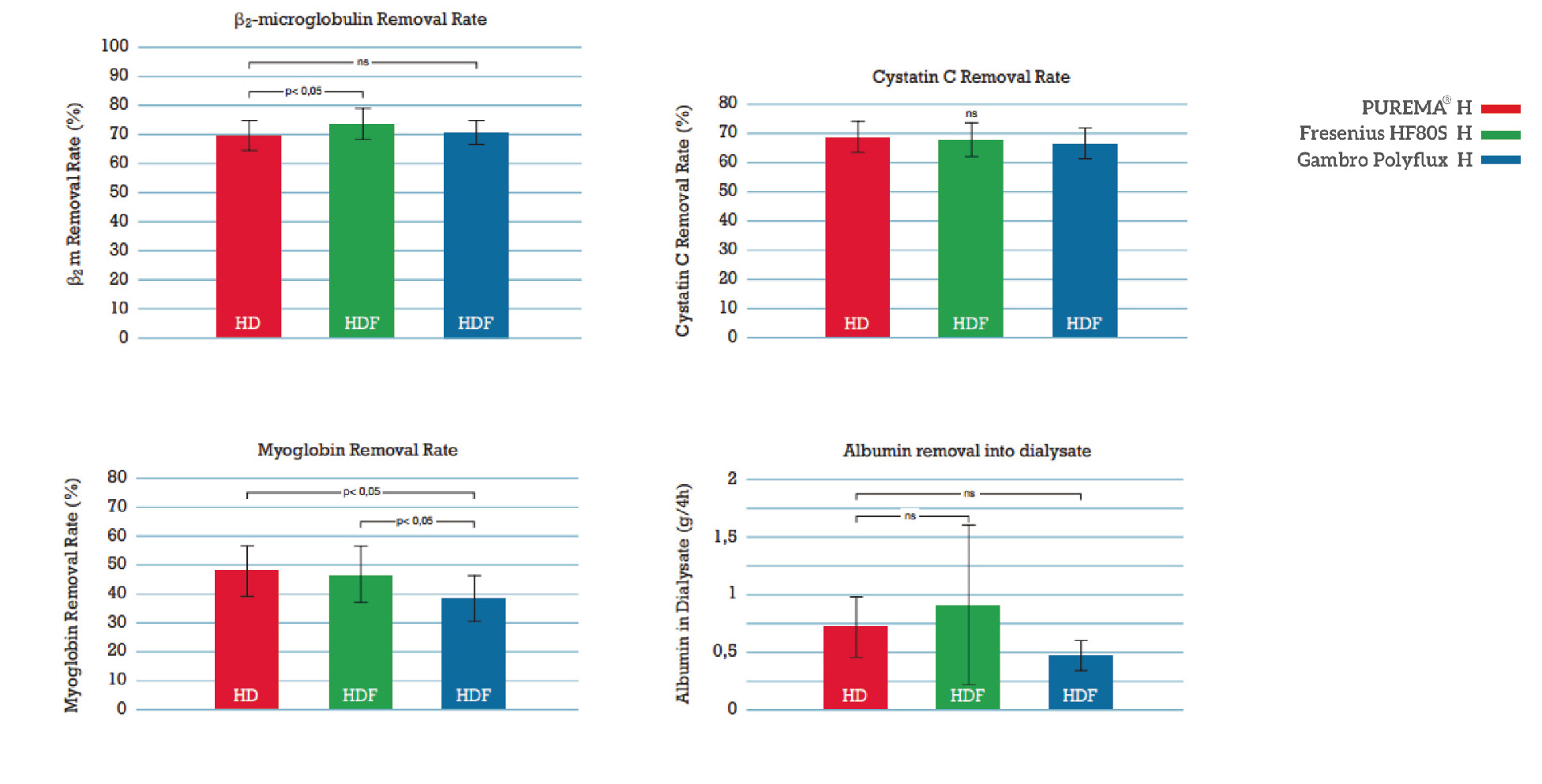
Excellent bio-compatibility
Significant endotoxin retention effects
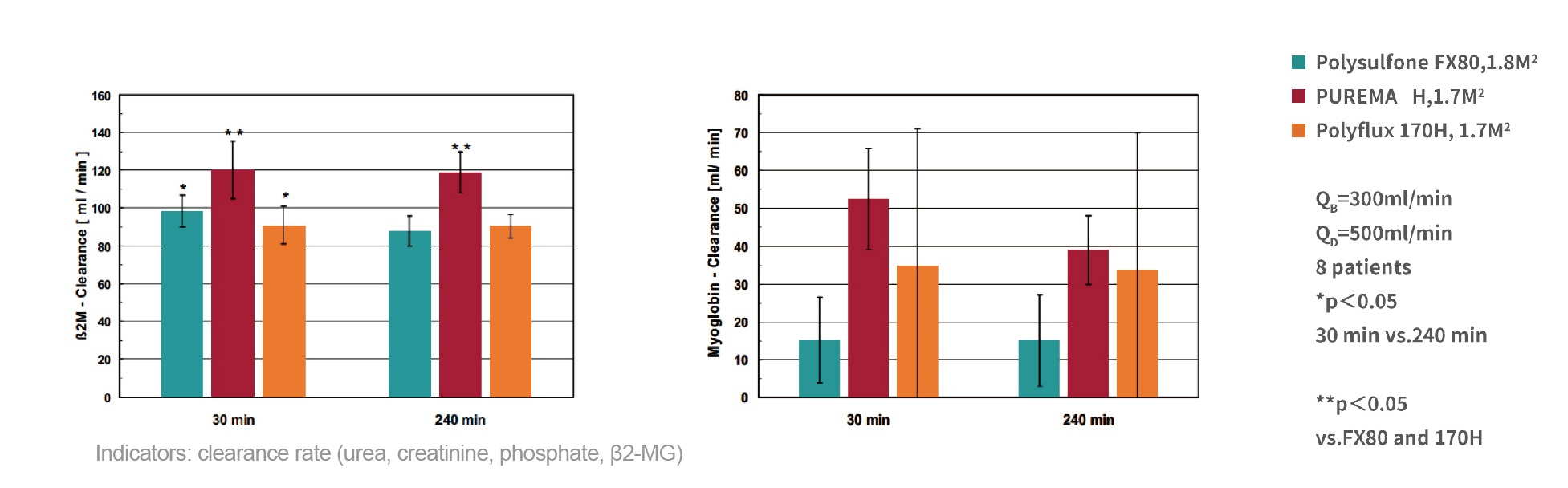
Consistent and efficient toxin removal performance
Efficient medium and large molecule removal efficienc
Excellent bio-compatibility
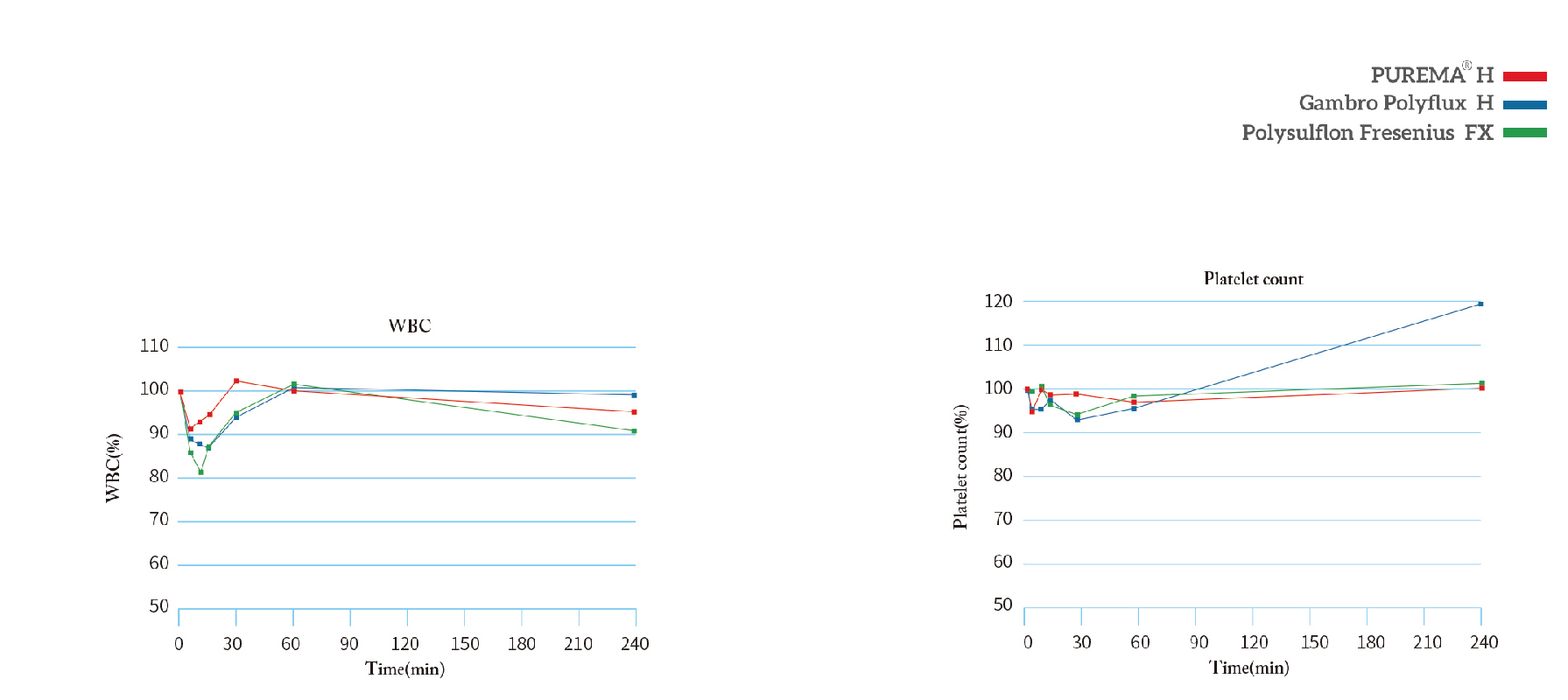
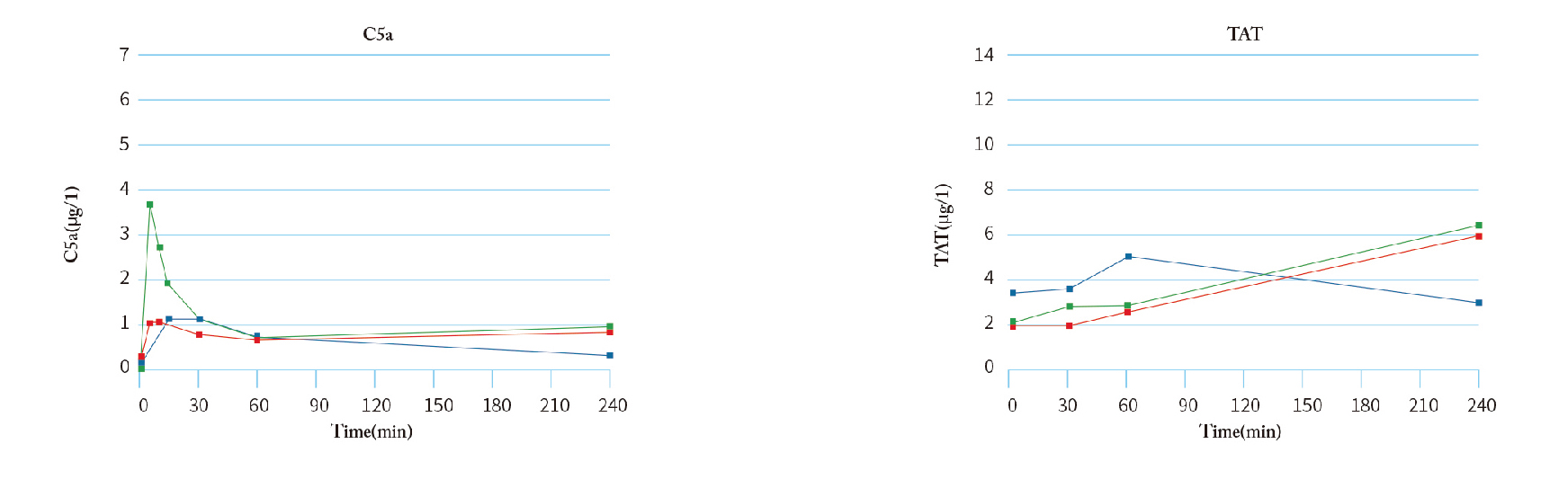
Less likely to cause changes in complement activation and leukocyte count, with better bio-compatibility.
Leukocytes: relatively stable; complement: relatively stable;
Indicators: leukocytes, complement, platelet count, coagulation factor activation
Effect of interleukin retention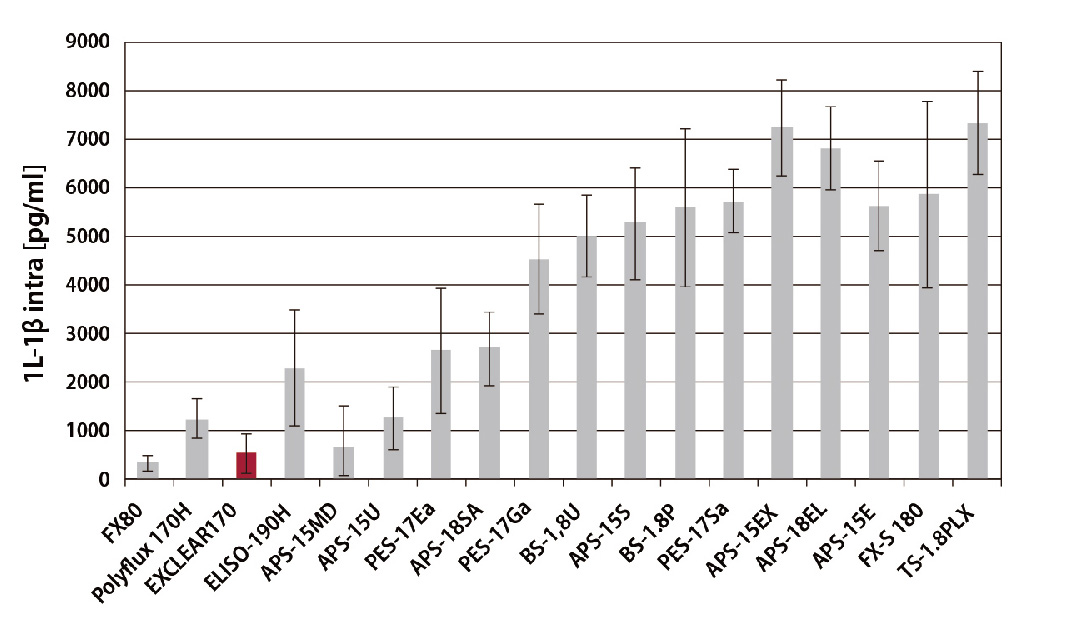
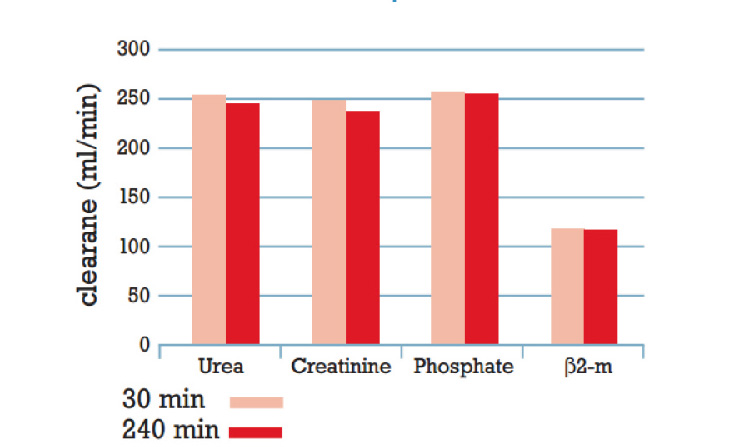
Stable clearance performance
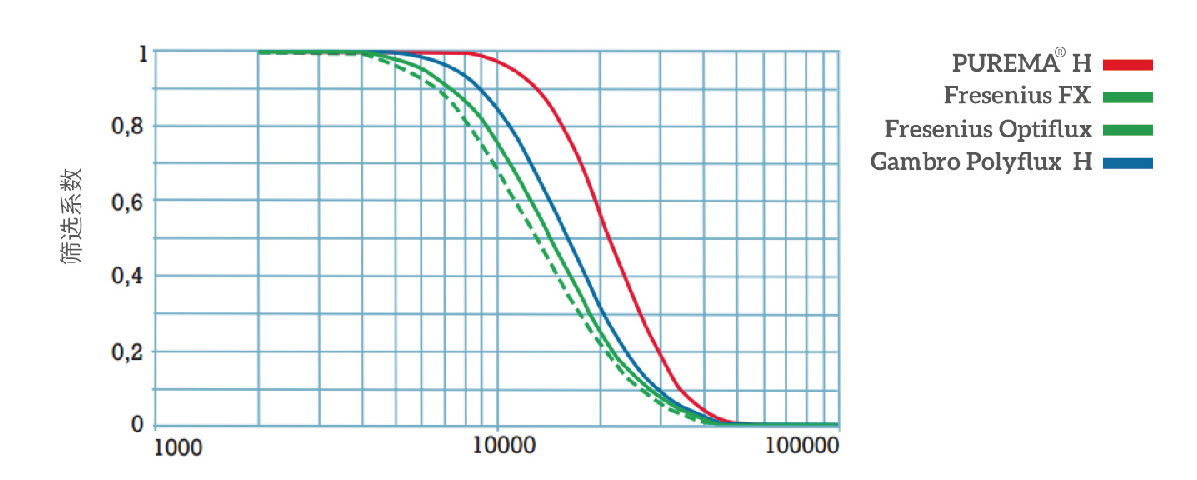
Rational distribution of screening coefficients
Efficient clearance rate of medium molecules
Comparison of high efficiency performance with control in HDF mode
Specification | EXCLEAR130 | EXCLEAR150 | EXCLEAR170 | |||
Kuf (mL/h-mmHg) | 38 | 41 | 45 | |||
CL | CL 200 (ml/min) | Urea | 192 | 195 | 197 | |
Creatinine | 184 | 188 | 191 | |||
Phosphate | 177 | 182 | 185 | |||
Vitamin B12 | 140 | 147 | 151 | |||
lnulin | 83 | 96 | 105 | |||
CL 300 (ml/min) | Urea | 260 | 271 | 276 | ||
Creatinine | 235 | 245 | 253 | |||
Phosphate | 220 | 230 | 240 | |||
Vitamin B12 | 160 | 174 | 185 | |||
lnulin | 93 | 108 | 118 | |||
CL 400 (ml/min) | Urea | 299 | 315 | 327 | ||
Creatinine | 273 | 282 | 293 | |||
Phosphate | 245 | 260 | 272 | |||
Vitamin B12 | 155 | 186 | 196 | |||
lnulin | 103 | 114 | 123 | |||
Area m2 | 1.3 | 1.5 | 1.7 | |||
Blood chamber volume (mL) | 82 | 97 | 106 | |||
Screen Factor | β2 Microglobulin | 0.7±0.1 | ||||
lnulin | 0.9±0.1 | |||||
Myoglobin | 0.5±0.1 | |||||
Albumin | < 0.01 | |||||
In vitro test conditions
CL:QD=500mL/min;QF=0mL/min
Kuf using bovine plasma with a protein concentration of 60g/L±5g/L,TMP 50mmHg.
Note: Different test conditions and techniques may yield different values.
Membrane wall thickness | 30µm | Membrane material | PES |
Membrane inner diameter | 200µm | Shell material | Polycarbonate |
Sterilization method | Radiation sterilization | Packaging material | Polyurethane Adhesive |
Package Qty | 12pcs/box | Gasket | Medical Silicone |