Shinva Medical - Yingde Bio was authorized as the first Siemens Headquarter MES Solution Partner in China's Pharmaceutical Industry
In December 2024, Yingde Bio-pharm Equipment Technology Co., Ltd., a subsidiary of Shinva Medical, successfully signed the Opcenter Execution Pharma Solution Partner agreement with Siemens with the support of VM Pharma and Phama Headquarter, the global headquarters of Siemens. With the support of this agreement, Yingde Bio has received the full-featured Opcenter EX PH software package for training, testing and laboratory development. Yingde Bio has become the first and only Opcenter EX PH SOP partner in China, besides Europe and the United States, authorized and certified by Pharma's pharmaceutical industry headquarter, and has gone live on the Xcelerator platform. By obtaining the authorization of MES Solution Partner and combining the industry experience, technical strength and market influence of the two companies, Yingde Bio will surely make a greater contribution to unmanned and paperless production and smart manufacturing in China's pharmaceutical industry.
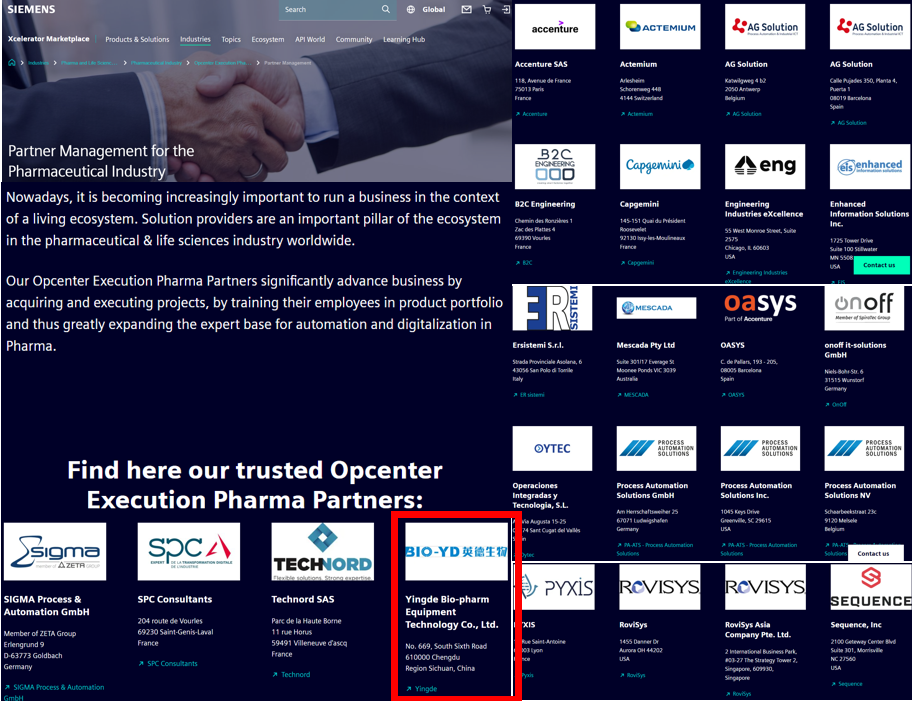
Yingde Bio is a leading system integrator and process equipment supplier in the biopharmaceutical field in China, and is also a platinum system integrator of Siemens Digital Industry Group, with a long-term and stable relationship.

In recent years, Yingde Bio has been improving its strength in the field of automation and informatisation, and has successfully delivered a large number of projects using fully integrated Siemens solutions such as PCS7+BATCH and PLC+WINCC+PM addon for its customers. Based on the needs of IOT convergence and the latest regulations, Yingde Bio has also taken the promotion and implementation of pharmaceutical MES system as one of the key areas of expansion in recent years.
