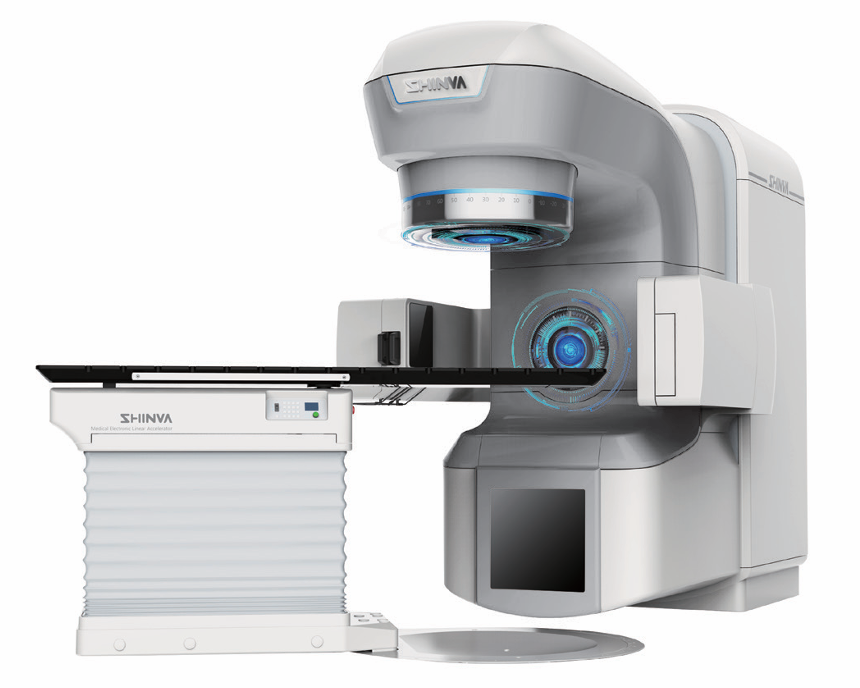
Shinva Secures the First MDR CE Certification for China Made Medical Linear Accelerators
Shinva’s self-developed medical linear accelerators, XHA600E and XHA1400, have recently obtained CE certification under the EU Medical Device Regulation (MDR). This marks the first time a China-made medical linear accelerator has been certified under the MDR framework, underscoring Shinva’s advanced technological capabilities in high-end radiotherapy equipment.
Notably, the XHA1400 has become the only medium-energy medical linear accelerator currently MDR-CE certified in the EU, granting Shinva a critical entry pass into one of the world’s most demanding medical device markets.
Meeting the Highest Regulatory Standard: The Significance of MDR Certification
The EU Medical Device Regulation (MDR, EU 2017/745) is widely regarded as the strictest regulatory system for medical devices worldwide. Compared with the former MDD, MDR imposes more rigorous requirements for clinical evidence, post-market surveillance, risk management and traceability.

For high-risk, technologically complex radiotherapy systems such as medical linear accelerators, MDR certification signifies that the manufacturer’s quality system, clinical data, and full-lifecycle product management meet leading global standards.
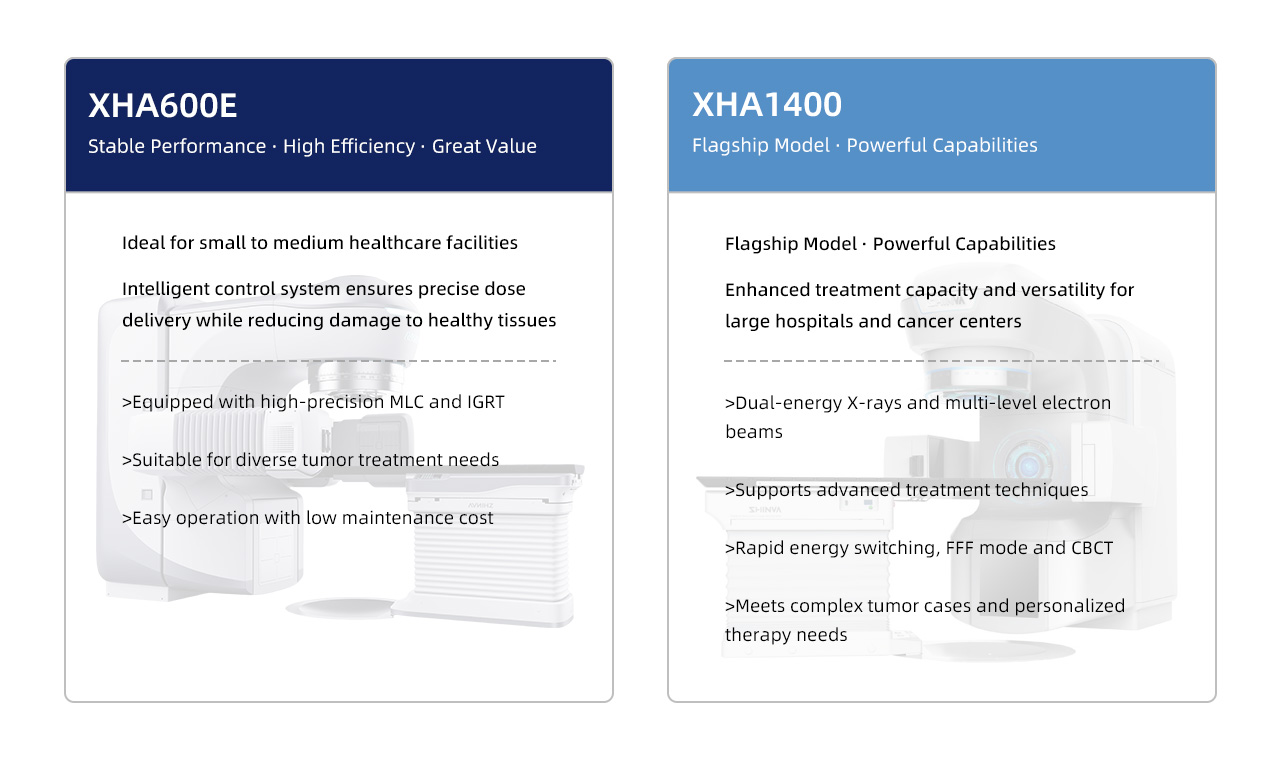
Two Flagship Systems Driving Precision Radiotherapy
The newly certified XHA600E and XHA1400 are designed to address precision radiotherapy needs across different tiers of healthcare institutions.
Market Outlook: Unlocking Europe’s Growth Potential
According to the European Society for Radiotherapy and Oncology (ESTRO), the EU market for medium-energy linear accelerators is valued at approximately EUR 450 million annually. MDR-CE approval clears the way for Shinva’s products to enter all 27 EU member states as well as Switzerland, the UK and other aligned markets.
This milestone is expected to generate significant overseas revenue growth for Shinva in the coming years and further strengthen its global brand presence. Securing the first MDR CE certificate for a China-made linear accelerator marks a historic breakthrough, demonstrating that Shinva’s products meet the highest international standards of safety, effectiveness and reliability.

Moving forward, Shinva will build on this achievement to deepen localized operations worldwide and deliver more “China solutions” not only to the EU, but also to Belt and Road regions and RCEP partner countries.
For more information
Learn more about SHINVA’s MDR-CE certified linear accelerators:
XHA600E: https://shinva.com/XHA600E-Medical-Electron-Linear-Accelerator
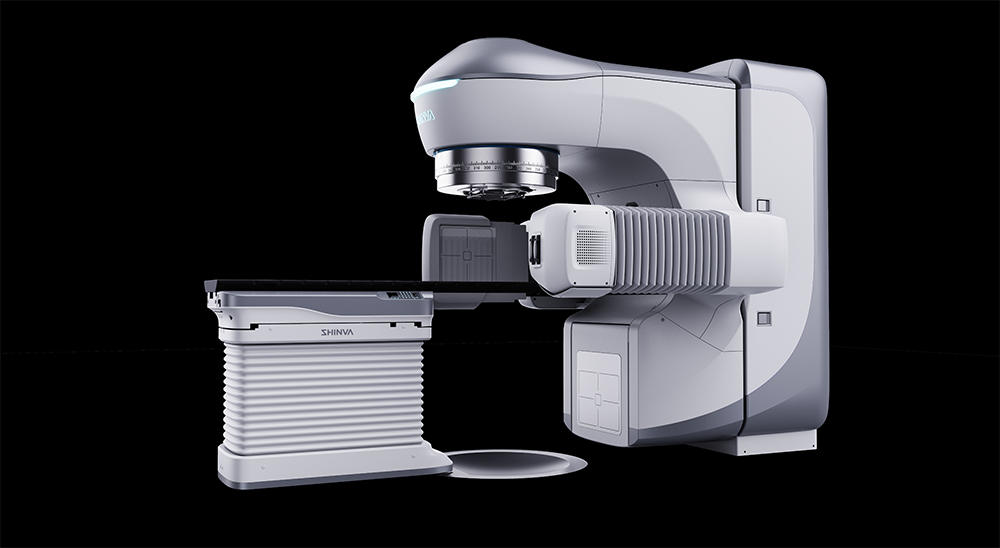
XHA1400: https://shinva.com/XHA1400-Medical-Electron-Linear-Accelerator